Current cancer therapies are generally limited to surgery, radiation, immunotherapy, and chemotherapy. While effective in many cases, all three of these therapies have limitations such as side effects and damage to surrounding normal tissue. The recent advent of nanotechnology may help mitigate these limitations. Nanotechnology could potentially be used, in conjunction with traditional cancer therapies, as a novel and precise targeting system. This increased targeting precision offers the possibility of eradicating tumors with little or no damage to surrounding healthy organs and tissues.
Through its selective targeting, nanotechnology promises to improve the pharmacokinetics and tumor specificity of chemotherapies. Chemotherapeutics can be either encapsulated or conjugated to the surfaces of nanoparticles (that are comparable in size to biologically relevant molecules).
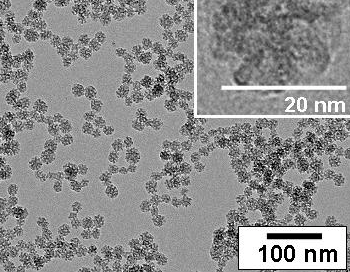
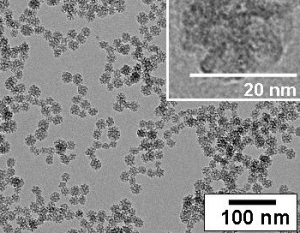 Transmission electron micrographs of 20nm silica nanoparticles.
Transmission electron micrographs of 20nm silica nanoparticles.
The most basic form of delivery for nanoparticle-based cancer therapies to tumors relies on a phenomenon known as the enhanced permeability and retention (EPR) effect. This effect is because tumors grow at a rapid pace, and hence the resulting vasculature often has relatively large pores and lacks structural integrity. These pores allow high concentrations of nanoparticles to enter and accumulate in the tumor as compared with surrounding tissue.
While, the EPR approach to nanoparticle delivery is effective as a cancer treatment, more specific approaches have also been developed. Nanoparticles can be linked to different biological molecules that can direct the nanoparticles to specific tumor cells. Tumor marker proteins are some commonly used tumor-targeting molecules.
Dr. Eggehard Holler’s lab, at Cedars-Sinai Medical Center, studies tumor markers as targets for nanotherapy and their 2014 JoVE article titled, “Polymalic Acid-based Nano Biopolymers for Targeting of Multiple Tumor Markers: An Opportunity for Personalized Medicine?” highlights their methodology for producing nano-drugs.
 Dr. Eggehard Holler, Cedars Sinai Medical Center
Dr. Eggehard Holler, Cedars Sinai Medical Center
An alternative targeting approach is coupling nanoparticles to monoclonal antibodies raised against tumor-specific plasma membrane epitopes. Dr. Konstantin Sokolov’s lab, at M.D. Anderson Cancer Center, highlights this approach in their JoVE article titled, “Synthesis of Immunotargeted Magneto-plasmonic Nanoclusters”.
 Dr. Konstantin Sokolov, MD Anderson Cancer Center
Dr. Konstantin Sokolov, MD Anderson Cancer Center