In this blog post, we will delve into the determination of melting points, a fundamental technique used to identify and analyze the properties of chemical compounds. Understanding a substance's melting point is crucial as it marks the specific temperature at which it transitions from a solid to a liquid under standard atmospheric pressure.
What is the Determination of Melting Points?
The determination of melting points is a fundamental technique used to identify and analyze the properties of chemical compounds. The melting point is the specific temperature at which a substance changes from a solid state to a liquid state. This transition occurs under standard atmospheric pressure, making it a critical characteristic alongside other physical properties like solubility, density, and color.
Understanding a compound's melting point helps chemists and researchers identify its material and verify its purity, which is particularly important in applications such as drug formulation and quality control in manufacturing.
How to Determine Melting Point in Chemistry?
Determining the melting point of a compound involves several steps, designed to ensure accuracy and repeatability. This process is crucial in educational labs for teaching students about physical properties and in professional labs for substance identification and quality control.
Melting points are more than just numbers. They provide insight into the chemical structure of a molecule and the strength of the forces holding it together. High melting points indicate strong intermolecular forces within the solid and a stable, tightly packed molecular structure.
Step-by-Step Guide to Melting Point Determination
Watch all related Melting Point Video at JoVE.com
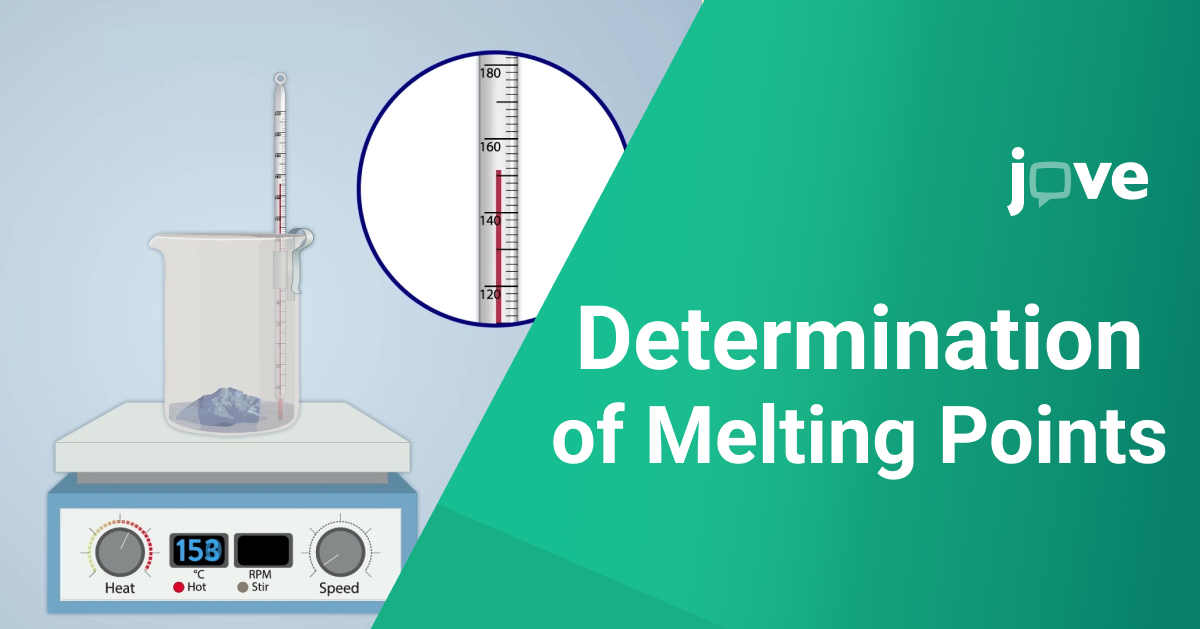
1.Preparation of the Sample: Start by grinding the compound into a fine powder. This ensures that the sample heats uniformly, preventing any part of it from melting prematurely, which could skew the results.
2.Loading the Capillary Tube: Fill a thin, transparent capillary tube with a small amount of the powdered compound. The tube should be no more than a few millimeters in diameter, allowing for quick and even heating.
3.Setting Up the Melting Point Apparatus: Insert the filled capillary tube into a melting point apparatus, a specialized device that slowly heats the sample while allowing clear observation. Make sure the tube is properly aligned with the apparatus's viewing window.
4.Heating the Sample: Increase the temperature at a controlled rate. This is critical to prevent superheating, where the temperature rises too quickly and does not accurately reflect the melting point.
5.Observing the Change: Carefully watch the sample as the temperature increases. Note the temperature at which the first signs of melting appear—the substance begins to turn from solid to liquid. Continue monitoring until the entire sample is liquefied.
6.Recording and Analyzing Results: Record the temperature range from when melting begins to when it completes. Compare this range to standard values available in scientific literature to assess the compound's identity and purity.
7.Repeating for Accuracy: Perform the test multiple times to ensure consistency and reliability in your results.
Intermolecular Forces and Melting Points
Melting points of compounds are significantly influenced by the types of intermolecular forces present among their molecules.
- Hydrogen bonding, a strong type of intermolecular force, typically occurs between a hydrogen atom and a highly electronegative atom like oxygen or nitrogen. This results in compounds such as water and alcohols having higher melting points compared to those where these bonds are absent.
- Dipole-dipole interactions, involving attractions between oppositely charged ends of polar molecules, also elevate melting points, as seen in substances like HCl or sulfur dioxide.
- Meanwhile, London dispersion forces, which are the weakest and arise from temporary dipoles in molecules, impact substances like noble gases and nonpolar hydrocarbons, resulting in generally lower melting points. These differences highlight the substantial energy required to overcome these forces during the melting process.
Interactive Learning with JoVE's Videos
JoVE's Melting Point educational videos are a cornerstone for demystifying complex scientific concepts, such as melting point determination. These resources use visual demonstrations and real-life applications to illustrate theoretical principles, making the learning process more engaging and effective. By viewing these experiments and their outcomes, students and professionals alike can gain a deeper understanding of the practical aspects of melting point analysis, which textbooks alone cannot provide.
Conclusion
Understanding the principles of melting point determination is crucial for anyone involved in chemical research and pharmaceutical development. Mastery of this technique is not just about academic knowledge; it is about enhancing the quality of scientific inquiry and ensuring the reliability of the results in practical applications. With resources like JoVE, learners and researchers can thoroughly grasp and apply these essentials, paving the way for advancements in science and technology.